Introduction: The standard treatment approach for DLBCL has traditionally relied on chemo-immunotherapy with R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone) for decades. However, approximately 30-40%, remain refractory or experience relapse with poor outcomes. Since 2017, the landscape for relapsed refractory DLBCL has changed dramatically with the FDA approval of newer monoclonal antibody, chimeric antigen receptor (CAR) T-cell, antibody-drug conjugate, and bispecific T-cell engagers. Nonetheless, a comprehensive analysis of population survival outcomes since the new treatment modalities remains to be evaluated. In this study, we present an extensive demographic analysis of one of the largest DLBCL cohort to date within the VHA, spanning over 11 years, and the survival outcomes before and after 2017 period. By assessing this vast dataset, we aim to provide valuable insights into the changing landscape of DLBCL treatment and its impact on patient survival.
Methods: Using the VHA electronic database, 6266 patients with an ICD code for DLBCL were randomly selected for this retrospective chart review from 01/01/2011, to 12/31/2021. Diagnosis outside of these dates or lack of any treatment history were excluded from the study. Patients were separated based on the diagnosis date into pre- and post-2017 era, diagnosis prior to 1/1/2017 vs diagnosis on and after 1/1/2017. Baseline disease characteristics, treatment patterns and outcomes were collected manually by trained abstractors. Finally, we calculated the overall survival (OS) and graphed using Kaplan Meier method comparing the pre- and post-2017 era. P-values were calculated using Log-Rank test or Chi-Square test.
Results: 2660 patients were included in the final analysis. Baseline characteristics between the two groups were comparable. Of all patients, 73% achieved complete response (CR) to the first-line treatment. Most of the patients (1968 patients, 74%) received CHOP-based therapy as their first-line regimen with a median of 6 cycles, and this did not differ significantly between the pre- and post-2017 era. Diagnostic lumbar puncture was performed in 19% of all patients, and about 14% of the patients received CNS prophylaxis. Only 4.2% of all patients proceeded with hematopoietic stem cell transplantation (HSCT). Notably, patients diagnosed post-2017 had fewer HSCT than pre-2017, 2.7% vs 5.1% respectively.
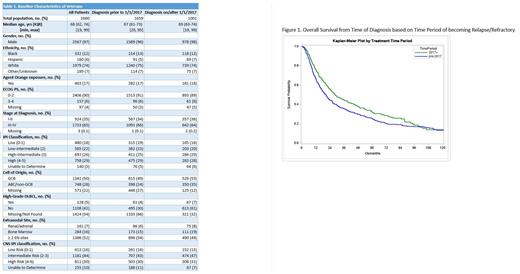
The median overall survival of all patients in the pre-2017 group was 96 months whereas post-2017 was not reached yet (P-value 0.011). Further analyzing the outcomes of the veterans who experienced refractory or relapsed disease, based on the date refractory or relapsed disease diagnosis, we identified improved OS for an extended period in the post-2017 group. The median OS of pre-2017 was 21 months vs post-2017 was 28 months (P-value 0.013).
The OS rate at 12 months and 60 months was 66% vs 76% (P-value 0.003) and 25% vs 32% (P-value 0.034) in the pre-2017 and post 2017 era respectively. However, the OS rate at 96 months was 17% vs 19% was not different statistically (P-value 0.48).
Conclusions: This is the most extensive DLBCL population-based data set existing, encompassing survival outcomes over a decade. Our study shows improved OS in patients diagnosed with refractory and relapsed disease on 1/1/2017 and beyond compared to the pre-2017 era, highlighting the available newer treatments outside the rituximab-based chemo-immunotherapy and refined supportive care. The improved survival extends to over 5 years. However, the overall survival remains similar after 8 years, irrespective of treatment era. This finding suggests that other factors, such as comorbidities, appear to play a role in mortality, rather than the disease itself or its treatment. The limitation of the study includes limited follow up period for patients diagnosed post 2017 era and the predominantly male population found in the VHA system, which represents a restricted view of a real-world population.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal